Oh What a Difference a Carbon Can Make
viii.14: Alcohols
- Page ID
- 49462
Molecules of alcohols contain 1 or more hydroxyl groups (OH groups) substituted for hydrogen atoms along the carbon chain. The construction of the simplest booze, methanol (methyl alcohol), tin be derived from that of methane by putting an OH in place of one of the H's:
| Methyl hydride | Methanol |
|---|---|
The name, too, is derived from the name marsh gas by replacing the final eastward with ol (for alcohol). The general formula for an alcohol may be written as R—OH, where R represents the hydrocarbon (alkane) portion of the molecule and is called an alkyl grouping. In methanol, R is the methyl group CH3.
Methanol is also called wood alcohol considering information technology tin be obtained past heating wood in the absence of air, a procedure chosen subversive distillation. Methanol vapor given off when the wood is heated can be condensed to a liquid by cooling below its boiling betoken of 65°C. The consequence of polarity and especially hydrogen bonding due to the OH group is evident when this is compared with the temperature of –85°C at which ethane, C2Hvi, boils. Both molecules comprise 18 electrons and are nearly the same size, and so London forces should exist virtually the same, but the OH group in one methanol molecule can grade stiff hydrogen bonds with an OH in another molecule. Methanol is an important industrial chemic—nearly 3 × 1010 kg was produced worldwide in 2003[1]. Some was fabricated by subversive distillation, simply most was synthesized from hydrogen and carbon monoxide:
\[\ce{2H_{ii} (g) + CO (one thousand) \longrightarrow CH_{3}OH(l)}\]
This reaction is carried out at pressures several hundred times normal atmospheric pressure, using metallic oxides as catalysts. Methanol is mainly used to make other compounds from which plastics are manufactured, but some is consumed as fuel in jet engines and racing cars. Methanol is too a component of nonpermanent antifreeze and automobile windshield-washer solvent.
The second fellow member of the alcohol family is ethanol (ethyl booze)― the substance we commonly call alcohol. Ethanol is likewise known as grain alcohol because it is obtained when grain or carbohydrate ferments. Fermentation refers to a chemical reaction which is speeded upwards by enzymes and occurs in the absenteeism of air.
Ethanol can likewise be synthesized by calculation H2O to ethene, obtained during petroleum refining:
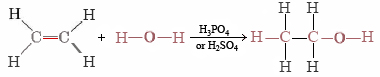
This is a typical case of an improver reaction. The H and OH from H2O are added to the ethene molecule and held there by electrons made bachelor when 1-half of the double bond breaks.
Ethanol is used as a solvent, in some special fuels, in antifreeze, and to manufacture a number of other chemicals. You are probably most familiar with it as a component of alcoholic beverages. Ethanol makes up 3 to 6 percentage of beer, 12 to 15 per centum of nigh wines, and 49 to 59 percent of distilled liquor. (The "proof" of an alcoholic beverage is merely twice the per centum of ethanol.) Alcohol'due south exhilarant effects are well known, and it is a mild depressant. Prolonged overuse can pb to liver damage. Methanol also produces intoxication but is much more poisonous than ethanol—it can cause blindness and expiry. Denatured alcohol is ethanol to which methanol or another poison has been added, making it unfit for homo consumption. Most of the ethanol non used in alcoholic beverages is denatured because in that form its sale is taxed at a much lower rate.
Two isomers are possible for alcohols containing iii carbon atoms:
| The ii strutural isomers of propanol, i-propanol and two-propanol(isopropyl alcohol) |
The i and the ii in the names of these compounds indicate the position of the OH grouping along the carbon concatenation. The propanols are much less important commercially than methanol and ethanol, although two-propanol is ordinarily found in rubbing alcohol.
A carbon atom typically forms iv bonds. Therefore, in an alcohol where carbon is bonded to an -OH group, at that place can be upwards to 3 carbon atoms directly bonded to the carbon atom bonded to the oxygen in -OH. If no carbon cantlet or 1 carbon atom is bonded directly, the compound is a chief alcohol. If two are bonded directly, information technology is a secondary booze; with three it is a tertiary booze, equally illustrated below.
| These three molecule are representative of a chief alcohol (1-butanol), secondary alcohol (2-butanol) and a 3rd booze (ii-methyl-2-propanol). |
All alcohols tin can exist completely oxidized to carbon dioxide and water by oxygen in the air; that is, all alcohols are combustible. Like hydrocarbons, combustion is an important reaction of alcohols, only more controlled oxidation is fifty-fifty more important, considering it can convert alcohols into other compounds that are useful to order. The ease with which an alcohol can be oxidized and the extent of the oxidation depends on whether the alcohol is primary, secondary, or tertiary.
For master alcohols, controlled, stepwise oxidation first yields compounds called aldehydes; if more than of the oxidizing agent is available, and then aldehydes can be further oxidized to carboxylic acids. Schematically
Primary alcohol → aldehyde → carboxylic acid
Oxidation of an organic compound tin can usually be recognized because either an oxygen cantlet is added to a molecule or ii hydrogen atoms are lost from a molecule. For example, stepwise oxidation of ethanol get-go produces the aldehyde ethanal (usually called acetaldehyde); further oxidation produces the carboxylic acid, ethanoic acid (commonly called acerb acrid). An aldehyde has the functional group –CHO, where the carbon atom is double-bonded to an oxygen cantlet. A carboxylic acrid has the functional grouping –COOH, in which the carbon atom is double-bonded to an oxygen atom and single-bonded to an oxygen cantlet in an OH grouping. The structures below bear witness the differences between ethanol, ethanal, and ethanoic acid.
| when oxidized gives and further oxidation gives |
Note that acetaldehyde differs from ethanol by loss of one H atom from the oxygen atom and ane H atom from the carbon on the correct. Acetic acid differs from acetaldehyde by having an add-on O atom on the right-hand carbon atom.
Controlled oxidation tin be carried out in the laboratory using an aqueous solution of potassium permanganate or an aqueous solution of potassium dichromate. When a similar controlled oxidation is applied to a secondary alcohol, such as 2-propanol (see beneath), the oxidized molecule contains a C=O grouping that has two other carbon atoms fastened to the C atom. This >C=O group with two carbon atoms attached to the C is called a ketone.
 |
Again, note that in the ketone the number of hydrogen atoms is fewer by two than the number in the secondary booze. The oxidation corresponds with loss of 2 hydrogen atoms. Ketones are difficult to oxidize further, because there is no manner to add together another oxygen atom to the carbon cantlet in the >C=O grouping, nor is in that location a mode to remove hydrogen atoms from the C and O atoms in the >C=O group.
Third alcohols, which have no hydrogen atoms attached to the carbon that is bonded to the –OH grouping, are difficult to oxidize. If a master alcohol, a secondary alcohol, and a tertiary alcohol are dissolved in water in three beakers and so treated with either potassium permanganate or potassium dichromate, merely the primary and secondary alcohols will react. (The reaction can exist observed because both permanganate ions and dichromate ions are colored (purple and orangish, respectively). Thus for the master and secondary alcohols, the colour will disappear, just for tertiary alcohols in that location will be no color change.
The structures of ii more alcohols which are of commercial importance and are familiar to many persons are shown below:
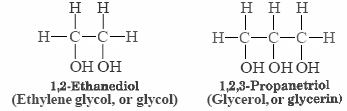
Each ethylene glycol molecule has ii hydrogens which tin participate in hydrogen bonding, and each glycerin molecule has 3. Both substances have rather high boiling points (198°C for ethylene glycol and 290°C for glycerin) and are syrupy, sticky liquids at room temperature. Their resistance to flowing freely is due to the network of hydrogen bonds that links each molecule to several of its fellows, making information technology more hard for them to slide past 1 another. This highlight again the consequence of hydrogen bonding on intermolecular forces and physical properties. In fact, in our tabular array of the boiling points of comparable organic compounds ethylene glycol has the highest boiling bespeak compared with other compounds containing the same number of electrons. The two propanol isomers are too on this table, only exceeded in boiling betoken by ethylene glycol, and acetic acid.
Ethylene glycol is the main component of engine coolant for automobiles and is also used to industry polyester fibers. In 2005, most one.viii × 1010 kg was produced worldwide. [2] Glycerin is used as a lubricant and in the manufacture of explosives:
When nitroglycerin is mixed with a solid cloth such as nitrocellulose (which is made by treating cotton or wood pulp with nitric acrid), the product is a class of dynamite.
References
- Pritchard, J.D. "Methanol - Production and Uses." Wellness Protection Agency. 22 October 2008. www.hpa.org.uk/web/HPAweb&.../1195733803581
- Pritchard, J.D. "Ethylene Glycol - Production and Uses." Wellness Protection Agency. fifteen October 2008. www.hpa.org.united kingdom/webw/HPAweb&am...=1190384322220
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Book:_ChemPRIME_%28Moore_et_al.%29/08:_Properties_of_Organic_Compounds/8.14:_Alcohols
